Health Care
324 products.
Filter by Application
[Close]
26 products.
[Close all]
Solutions for Pharmaceuticals
[Close]

Mitsubishi Chemical offers comprehensive solutions for medical devices and pharmaceutical packaging, leveraging its deep expertise and proven strong presence in the market over the years. We are expanding our global presence across a wide range of medical fields, providing high-performance resins, films, and tablet packaging for primary packaging in direct contact with pharmaceutical products. Additionally, we supply materials for single-use manufacturing components in pharmaceutical market. Our expert team is ready to recommend the ideal materials and technologies to suit your specific application. Please contact us today to learn more.
Polyvinyl Alcohol is a pharmaceutical excipient and listed in major official compendia.
It is widely used in many countries including Japan, the United States, and Europe.
Our GOHSENOL™ EG conforms to pharmacopoeia excipients including JPE*
1,USP*2 and EP*3
- *1Japanese Pharmaceutical Excipients (JPE)
- *2United States Pharmacopeia (USP)
- *3European Pharmacopoeia (EP)

Vinegar Powder Mixture is sodium acetate with slight flavor of acetic acid.It is used as a sour agent and pH regulator.
Vinegar Powder Mixture is our trade mark, categorized as composition of sodium acetate (anhydrous) and glacial acetic acid.
Composition: 58% of sodium acetate (anhydrous), 42% of glacial acetic acid
X-ray Scintillator Screen, DRZ™
[Close]

Scintillator is a material that convert radial ray such as X-ray into the visible light. By using with a light detector such as TFT, CCD, CMOS sensors, The image can be efficiently converted to a digital image.
Mitsubishi Chemical has its own phosphor manufacturing technology and processing know-how, including the DRZ series used for medical X-ray diagnostic imaging and baggage inspection, We have commercialized excellent scintillators for digital X-ray equipment such as Short Decay and Low Afterglow Type used for high-speed line sensors for contamination inspection.
Capable of advanced separation and purification in chromatographic separation of products thanks to smaller particle size than DIAION™ HP20 and SP207, which have excellent adsorption properties. This series consists of HP20SS, SP20SS with smaller particle size, and SP207SS.
MCI GEL™ packing materials for high performance liquid chromatography are synthetic polymer products for use as ion exchange resins, non-functional reverse-phase support, hydrophilic porous gels, etc. Particle size ranges from 4µm to more than 100µm. Quantities for analytical applications up to production-scale fractionation are available. We are prepared to supply materials such as ion exchange resins for glycoamino acid analysis, polymer adsorbent for reverse-phase chromatography, various column packings for protein separation, etc.
HPLC packed columns, MCI GEL
[Close]

These are HPLC separation columns that achieve high-resolution separations utilizing our proprietary column packing technology that features the high-quality MCI™ GEL packing material. MCI GEL packed columns serve application fields such as amino acid analysis, protein analysis, common organic compound analysis, and ion chromatography.
The Synthetic Adsorbents are cross-linked polymer spheres which provide specific surface area of 500-1200m2/dry-g with their porous structure. Available products include the aromatic-based HP series, the aromatic-based SP series, and the methacrylate ester-based HP2MGL. They are used for the adsorption purification of peptides, proteins, polyphenols, cephalosporin C, etc. as well as for the removal of bitter-tasting substances from fruit juices.
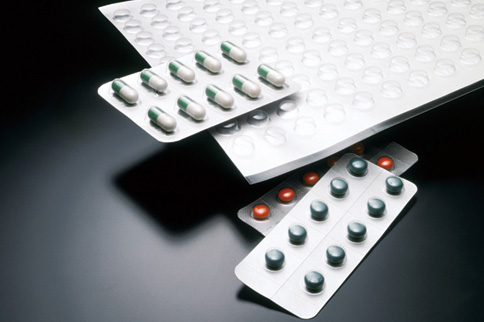
This plastic sheet is used to manufacture press-through packages (PTP) manufactured under Good Manufacturing Practices (GMP) required for medical packaging materials. This material complies with Notification 370 from the Ministry of Labour and Welfare issued in 1959, the Japan Hygienic PVC Association (JHP) standards, and Japan Hygienic Olefin and Styrene Plastics Association PL standards. This material is also registered in the US Food and Drug Administration (FDA) drug master file.
We use various testing equipment such as actual packaging machines used by the pharmaceuticals industry to ensure packaging properties of each material.
We are engaged in development to improve stability of pharmaceuticals manufacturing as well as incorporate the perspective of users including both patients and pharmacists.
- Product Finder
-




