
Implantable Engineering Plastics
Performance Materials and Technical Support
Power Advancements in Artificial Joints
October 6, 2023
/ TEXT BY MCG
*The information, positions and affiliations mentioned in this interview reflect the status at the time of the interview.
Artificial joint replacement is a surgical procedure to replace a joint that has been damaged or deformed due to disease, injury, or aging with a prosthesis, or implant. The procedure is designed to reduce joint pain, improve quality of life (QOL), and extend healthy life expectancy. As Japan's population ages, the number of joint replacement surgeries is increasing and is expected to continue growing. Against this backdrop, the Mitsubishi Chemical Group (or MCG Group), which supplies engineering plastics used in medical devices such as artificial joints, as well as surgical instruments and other apparatuses, is working to create value in medical settings by leveraging its advanced expertise and global network.

- Shimpei Nonoyama
Business Development Group, Sales Department
Mitsubishi Chemical Advanced Materials Japan - ■Medical and Healthcare
A member of the MediTECH Team engaged in business and application development, sales, and technical support for medical engineering plastics.
What are engineering plastics for implants?
The MCG Group handles two engineering plastic materials as MediTECH for implants: ultra-high molecular weight polyethylene (simply "polyethylene" below) and polyetheretherketone (PEEK).
Mr. Nonoyama said, "Polyethylene has excellent sliding properties and abrasion resistance. It's mainly used for liners that serve as cartilage in artificial joints. It helps hip and knee joints move smoothly and reduces wear, thereby reducing the risk of repeat surgeries and complications. Polyethylene is also easy to machine process, making it a suitable material for medical devices with complex shapes. PEEK, on the other hand, is an engineering plastic with good mechanical strength and elasticity similar to that of cortical bone. It is hard and is mainly used in spinal fusion cages to treat herniated discs and in anchor screws to affix ligaments to bone."
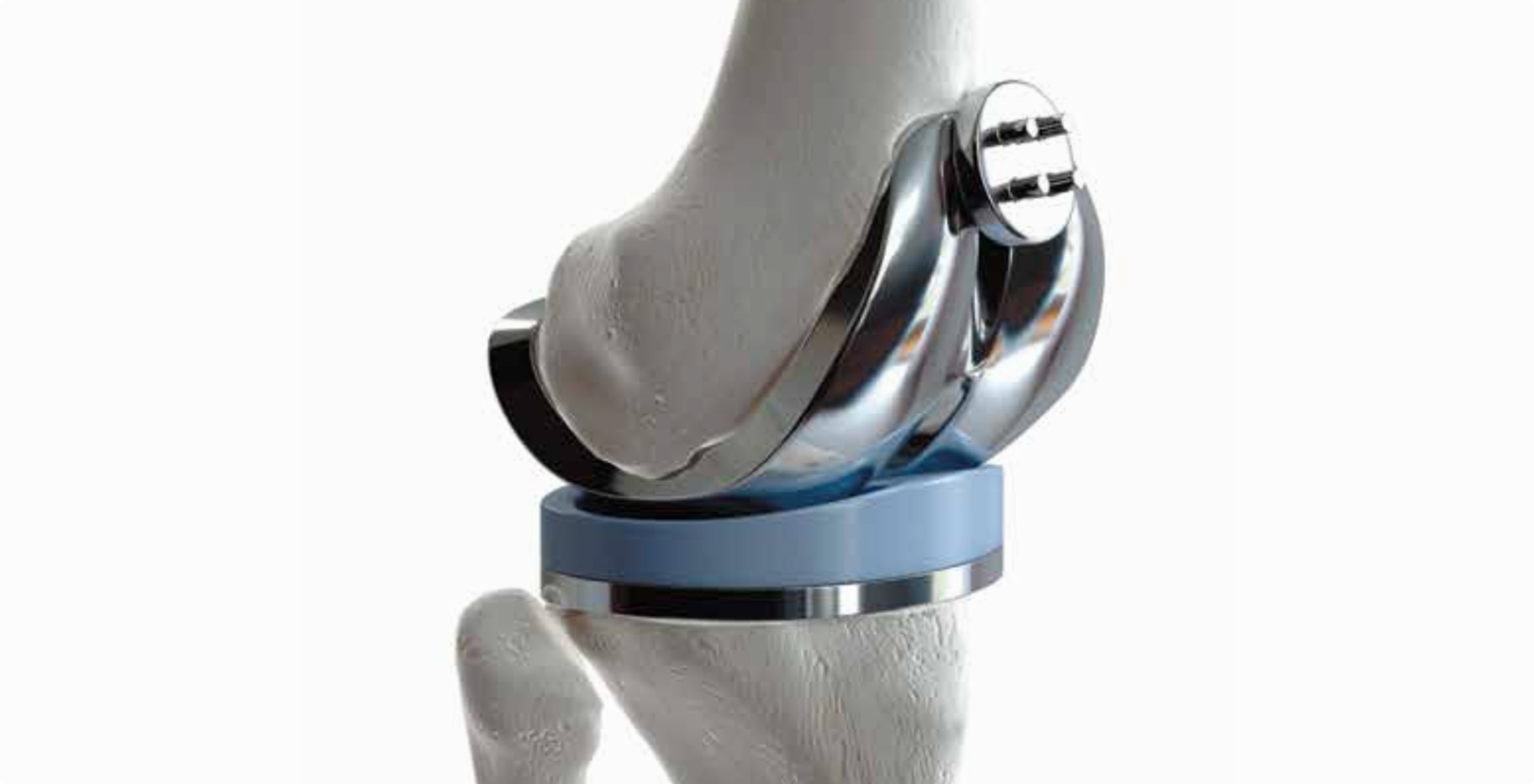
※Image of artificial knee joints
Generally, the service life of artificial joints was said to be 15 to 20 years, but university research institutes and medical device manufacturers have been conducting R&D to improve durability and have made numerous improvements in materials and shapes.
According to Mr. Nonoyama, "To prevent oxidation, which affects the service life of artificial joint liners, we add a small amount of vitamin E to the polyethylene, which is then cross-linked with radiation, such as gamma rays or electron beams. Our material design and processing technologies, including vitamin E blends, material molding methods, and cross-linking treatments that follow specifications, allow us to meet the needs of medical device manufacturers and help make their artificial joints more functional."
Thorough quality management
In medical materials, safety is the top priority. Medical devices that are implanted in the body for a long period require a great deal of time and money for the application and approval process before they can go on the market.
The MCG Group's medical engineering plastics have been pre-evaluated for biological safety at the medical level in accordance with the International Organization for Standardization (ISO) and United States Pharmacopeia (USP). MediTECH-grade production sites in North America and Germany have ISO 13485 certification, a quality management standard for medical device manufacturing. These sites perform thorough quality control and safety management during production.
Mr. Nonoyama said, "Assessing medical devices for safety is very important. Depending on the assessment item and testing method, there are different contact areas and time periods: 24 hours, within 30 days, and long-term in-body implantation. Usually, medical device manufacturers confirm the safety of their materials, but since our materials undergo that evaluation in advance, medical device manufacturers can simplify some safety tests based on those results, which should shorten the development schedules for medical device manufacturers.* To deliver better medical devices to medical professionals and patients as quickly as possible, we are endeavoring to keep up with development needs by delivering materials in a timely manner to improve and develop medical devices."
*Regulations and safety assessment details vary by country and region.
What are the advantages of a wide-ranging portfolio?
In addition to engineering plastics used for implants, the MCG Group supplies materials for instruments and medical devices used in surgery. For example, our Life Science-grade materials, which are used in medical devices intended for surgery (up to 24 hours) or for removal within 30 days, are used in fitting liners to check the size of an implant before implantation, surgical instruments, as well as parts for biopharmaceutical manufacturing equipment.
"The MCG Group's strength lies in its extensive portfolio, which allows customers to choose the material that best suits their purpose and application," said Mr. Nonoyama. He added, "There are manufacturers that specialize in either implant materials or medical materials, but our strength is that we can offer a one-stop shop for both. That ability to supply everything from implants to surgical instruments facilitates our customers' procurement and quality management. We believe that this enables our customers to maintain a certain level of quality, which leads to greater trust from the medical community."
New processing technologies bring new values
The MCG Group's strengths as a material manufacturer are its advanced engineering and global supply chain.
On the engineering side, the company is working on practical applications for Direct Compression Molding, or DCM. This is a new processing technology that will reduce costs and shorten working hours for medical device manufacturers in their production processes.
Since polyethylene is incompatible with injection molding, it is usually supplied in simple shapes formed through compression molding. Medical device fabricators machine medical device parts from these polyethylene lumps, but the leftover material goes to waste.
That's why the MCG Group has been developing DCM that employs the company's proprietary engineering to perform compression molding of complex shapes that still have the uniform and stable physical properties for medical use. Mr. Nonoyama said, "By delivering polyethylene in a form close to the final shape of the part to be manufactured by the customer, we can reduce machining waste by 30% to 40% and shorten working hours."
The MCG Group is now in the final stage of obtaining ISO certification for the DCM manufacturing process and will begin utilizing the technology in operations soon.

Technical support by experts
The MCG Group operates its engineering plastics business at 46 locations in 19 countries around the world. The company has formed the special MediTECH Team, which possesses a high level of medical expertise and knowledge.
The team's members are assigned to locations across the globe. "Due to their proximity to our customers, our personnel with in-depth knowledge of local medical regulations and resins can support customers' product development. This is a major competitive advantage for us," said Nonoyama.
The MediTECH team is also in a position to identify technology needs through communication with medical device manufacturers, physicians, and university research institutes. By sharing information obtained from medical professionals with group members around the world on what materials will become mainstream next and what medical needs there are, while fully utilizing the company's accumulated technologies and knowledge, the MCG Group can propose timely solutions to improve customers' products and develop the next generation of medical devices.

MediTECH team
Producing engineering plastics creates new potential for well-being
The MCG Group has contributed to the evolution of medical devices by providing a wide-ranging portfolio and technical support. As a manufacturer of materials for medical devices, the company's mission is stable production and quality control for polyethylene and other medical materials.
Engineering plastics as liners for artificial joints have already matured and established a solid reputation in the healthcare industry. Meanwhile, new challenges await, such as the need for active post-operative lifestyles because patients receiving these artificial joints are growing younger.
Mr. Nonoyama said, "One future challenge for artificial joints will be converting metal parts to resin. We also want to address the needs of the medical community with technology, such as material design that is custom-made or compatible with more precise 3D modeling. Outside of the orthopedics field, we are making progress on R&D for IoT medical devices, such as electroencephalographs and heart rate monitors that are implanted in the patient's body. The materials used in these devices must match the high level of safety and performance required for the device, so I believe MediTECH has great potential."
With materials experts that respond to emerging needs for materials in medical devices, the MCG Group is committed to supporting the development of medical devices that improve QOL for patients and is pursuing new possibilities in well-being for people through added value in medical settings.

MediTECH implantable polymers
Mitsubishi Chemical Advanced Materials
The Voice

- Dr. Teruya Ishibashi
Endowed Chair Assistant Professor of Orthopedic Biomaterial Science
Osaka University, Japan
■We asked Dr. Teruya Ishibashi, Endowed Chair Assistant Professor of Orthopedic Biomaterial Science at Osaka University, about artificial joints.
- Artificial joints today and the importance of materials
- With the aging society, every year we have an increasing number of patients suffering from osteoarthritis and other bone and joint problems. In Japan, approximately 100,000 knee arthroplasties and 70,000 hip arthroplasties are performed annually.
- Smooth movement and load-bearing support are important for a movable joint to function. The same is required of artificial joints. To ensure that artificial joints move smoothly to minimize wear, the choice of material, such as metal, polyethylene and ceramics, as well as the combination in which these materials are used, are important. Long-term durability, especially for artificial joints in the lower extremities, requires the strength to bear loads that are many times greater than the patient's body weight.
- In response to these challenges, the use of ultra-high molecular weight polyethylene, with its superior friction and abrasion resistance and durability, has extended the usable life of artificial joints. In addition, cross-linked polyethylene, which is improved by cross-linking with gamma ray treatment, and polyethylene containing vitamin E have further improved abrasion resistance.
- Further challenges to improving quality of life (QOL)
- These recent improvements in artificial joint materials and shapes, as well as accurate surgery using navigation and robot-assisted systems, have extended long-term durability beyond 20 years and ensured that joints function after operations. Meanwhile, a new challenge has emerged: subjective evaluations by patients on their expectations and satisfaction that are not very high.
- For example, there are patients who feel uncomfortable about having a artificial joint, or "machine," in their body, even if it eliminates any trouble they had walking or exercising. There are also patients who experience lower quality of life due to restrictions on playing sports after surgery. To address these challenges, researchers are constantly studying ways to improve surgical methods, implant materials, and shapes.
- The movement of the knee is a particularly complex combination of flexion and extension as well as axial rotation (twisting) and varus-valgus. Current artificial joints are unable to adequately reproduce these movements. In the future, we will need artificial joints with a three-dimensional structure that can guide such movements and withstand the friction and load between implants, so implant materials have to be easy to process and maintain their strength.
- In addition, custom-made implants are being developed to suit an individual's three-dimensional shape and movement to improve quality of life. I look forward to further advances in materials and closer collaboration with material manufacturers.









