- Mitsubishi Chemical Corporation
- Ion Exchange Resin Business Gr. Separation Materials Dept.
Weakly Basic Anion Exchange ResinsDIAION™ series
Mitsubishi Chemical Corporation
Characteristics
[Close]
Resins using the primary-ternary amino groups as functional groups behave like weak bases and are therefore called weakly basic anion exchange resins.
Because the weakly basic anion exchange resin has a weakly alkaline functional group, in basic solution it does not dissociate and therefore has no ion exchange ability.
Although the anion of a salt like NaCl or Na2SO4 cannot be exchanged, the anion of a mineral acid such as HCl and H2SO4, or the salt of a weak base like NH44Cl can be carried out as shown in the reactions below.
R-NH2+ HCl → R-NH3Cl
R-NH2+ NH4Cl → R-NH3Cl + NH3
Weak acid is generally hard to capture, and although there is a resin with which H2CO3 is exchangeable, silicic acid (silica) cannot be exchanged.
An advantage of the weakly basic resin is that it is easy to regenerate. Of course NaOH, but even Na2CO3, NH3, etc. can be used for regeneration. And this regeneration is economical as only slightly more than the theoretical chemical equivalent amount of regenerant solution is required.
Applications
[Close]
Applications include water treatment as well as purification of pharmaceuticals and food.
Usage
Packed column or Batch processing
Lineup / Specifications
[Close]
Mitsubishi Chemical's Weakly Basic Anion Exchange Resins are listed below.
| Substrate | Acrylic-based | Styrene-based Polyamine Type | Styrene-based Dimethylamine Type | |
|---|---|---|---|---|
| Product Name | WA10 | WA20 | WA21J | WA30 |
| Structure | 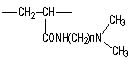 |
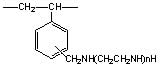 |
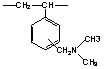 |
|
Refer to the "ION EXCHANGE RESINS "[別窓表示]websites for more details.
Inquiries Concerning Products
View the products of Separation Materials Dept., Mitsubishi Chemical[Open in a new window]
